Answer
409.2k+ views
Hint: Furanose is a collective term for carbohydrates that represents a chemical structure including a five-membered ring system consisting of four carbon atoms and one oxygen atom. Chemically furanose is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose.
Complete answer:
-Furanose ring consists of four carbon and one oxygen atom having an anomeric carbon to the right of the oxygen atom. The highest numbered chiral carbon determines whether the structure will have D-configuration or L-configuration.
-Furanose with an L-configuration has the substituent on the highest numbered chiral carbon which is pointed downwards out of the plane and in a D-structure furanose, the highest-numbered chiral carbon is facing upwards.
-Depending on the direction of anomeric hydroxyl group pointing, the furanose ring will either have alpha or beta configuration. In a D-configuration of furanose, if the hydroxyl group is pointing down then it is an alpha configuration and if the hydroxyl group pointing up then it is a beta configuration. Similarly, it is opposite with respect to an L-configuration.
-For converting a Fisher projection to a Haworth projection, we will follow the following steps-
(i) You first need to number carbons and draw in the Fisher stereochemistry.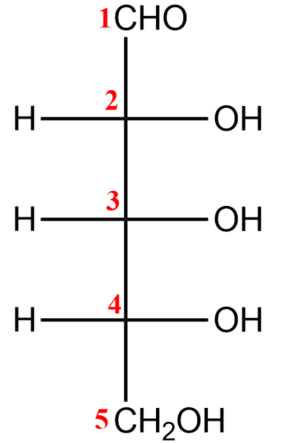
(ii) Now, rotate the molecule to $90{}^\circ $ in clockwise direction.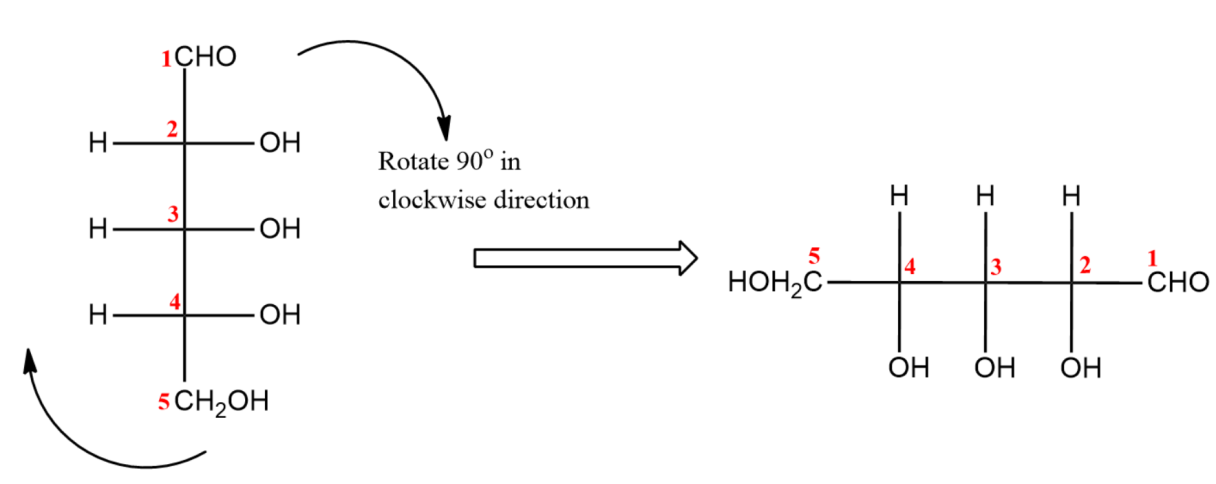
(iii) Next step is the bond rotation of ${{C}_{4}}$with ${{C}_{5}}$.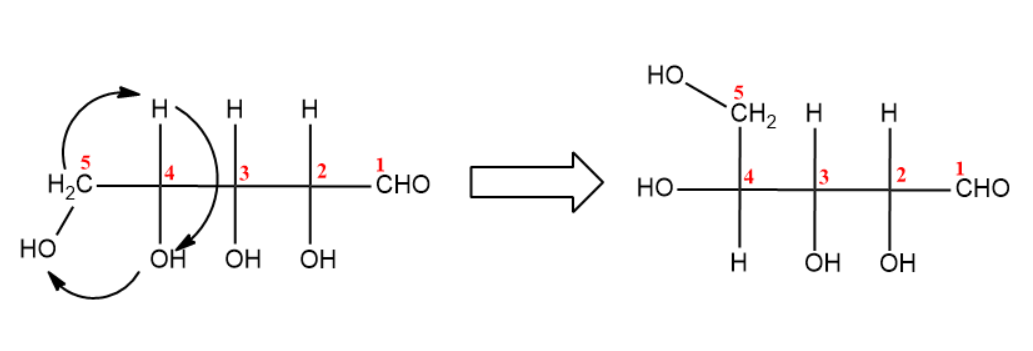
(iv) Last step is the closure of fischer projection forming a closed ring structure.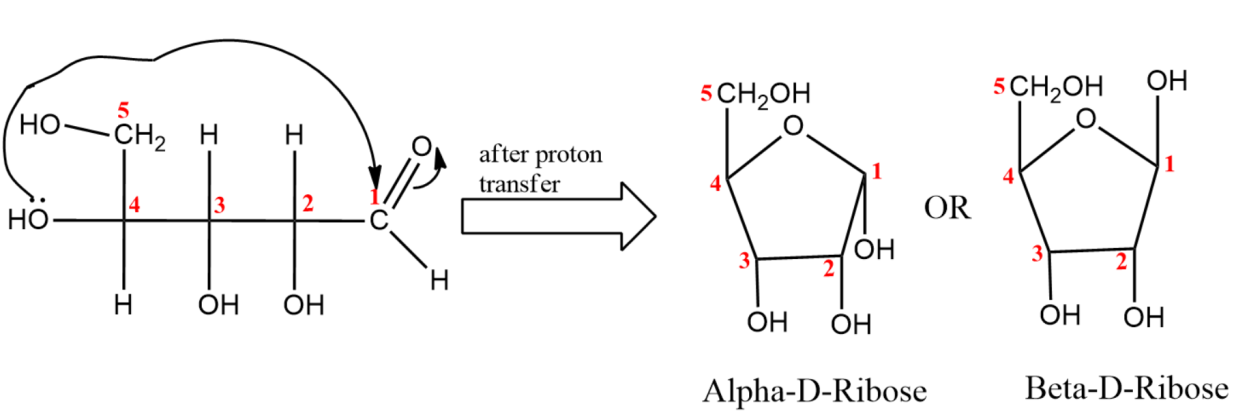
(2) In the next part of the question, we are asked to write Fisher projection of $\alpha -D$Ribulofuranose.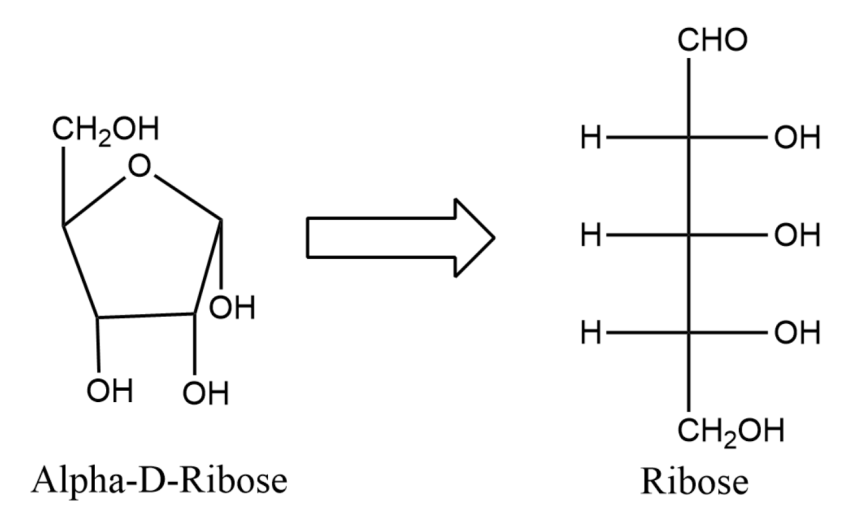
Note:
For D-sugars, the last carbon will end up at the top of the Haworth projection. For L-sugars, the last carbon will end up on the top of the Haworth projection. At ${{C}_{1}}$ for D-sugars, if the OH group is down then it is an alpha-anomer whereas if the OH group is up then it is a beta-anomer.
Complete answer:
-Furanose ring consists of four carbon and one oxygen atom having an anomeric carbon to the right of the oxygen atom. The highest numbered chiral carbon determines whether the structure will have D-configuration or L-configuration.
-Furanose with an L-configuration has the substituent on the highest numbered chiral carbon which is pointed downwards out of the plane and in a D-structure furanose, the highest-numbered chiral carbon is facing upwards.
-Depending on the direction of anomeric hydroxyl group pointing, the furanose ring will either have alpha or beta configuration. In a D-configuration of furanose, if the hydroxyl group is pointing down then it is an alpha configuration and if the hydroxyl group pointing up then it is a beta configuration. Similarly, it is opposite with respect to an L-configuration.
-For converting a Fisher projection to a Haworth projection, we will follow the following steps-
(i) You first need to number carbons and draw in the Fisher stereochemistry.

(ii) Now, rotate the molecule to $90{}^\circ $ in clockwise direction.

(iii) Next step is the bond rotation of ${{C}_{4}}$with ${{C}_{5}}$.

(iv) Last step is the closure of fischer projection forming a closed ring structure.

(2) In the next part of the question, we are asked to write Fisher projection of $\alpha -D$Ribulofuranose.

Note:
For D-sugars, the last carbon will end up at the top of the Haworth projection. For L-sugars, the last carbon will end up on the top of the Haworth projection. At ${{C}_{1}}$ for D-sugars, if the OH group is down then it is an alpha-anomer whereas if the OH group is up then it is a beta-anomer.
Recently Updated Pages
How many sigma and pi bonds are present in HCequiv class 11 chemistry CBSE

Why Are Noble Gases NonReactive class 11 chemistry CBSE

Let X and Y be the sets of all positive divisors of class 11 maths CBSE

Let x and y be 2 real numbers which satisfy the equations class 11 maths CBSE

Let x 4log 2sqrt 9k 1 + 7 and y dfrac132log 2sqrt5 class 11 maths CBSE

Let x22ax+b20 and x22bx+a20 be two equations Then the class 11 maths CBSE

Trending doubts
Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Which are the Top 10 Largest Countries of the World?

Write a letter to the principal requesting him to grant class 10 english CBSE

Difference between Prokaryotic cell and Eukaryotic class 11 biology CBSE

Give 10 examples for herbs , shrubs , climbers , creepers

Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE

Change the following sentences into negative and interrogative class 10 english CBSE

Difference Between Plant Cell and Animal Cell

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE






