Covalent bonding teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about covalent bonding (GCSE and Key Stage 3)
“A covalent bond is a shared pair of electrons”. But hang on a minute, surely a pair of electrons would repel each other and not form an attraction between two atoms. This makes no sense. Of course the covalent bond is far more than a shared pair of electrons and it’s important to stress that a covalent bond is actually the electrostatic attraction between the shared electron pair and the oppositely charged nuclei and vice versa. You may even want to show your students how the potential energy changes as you bring two nuclei together. It’s important to discuss both the attractive and repulsive forces when teaching covalent bonding.
Students will have different strategies to solve dot and cross diagrams and that’s fine – the key is to make sure they can make the link between the different representational forms of displayed formulas, structural formulas and dot and cross diagrams.
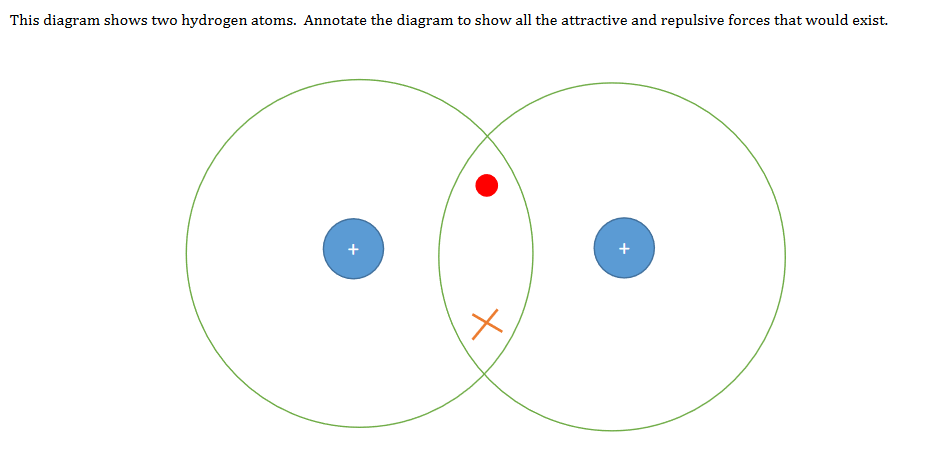 Drawing dot and cross diagrams for covalent molecules
Drawing dot and cross diagrams for covalent molecules
GCSE worksheet drawing dot and cross diagrams for covalent molecules. Students first have to consider the attractive and repulsive forces present when two atoms bond. Students then progress onto drawing dot and cross diagrams for increasingly complex molecules. (PDF)
GCSE covalent bonding challenge for drawing dot and cross diagrams! Students move around the room, drawing dot and cross diagrams for increasingly complex covalent molecules. Answers are on the back of each problem so students receive immediate feedback. This resource was contributed by Deborah Brown. (PDF)
Modelling covalent bonding
Molymods are a great way to help students conceptualise shapes of molecules and covalent bonding. If you use these models as a whole class make sure you train students first in how to use them. Explain what the different colours and sticks represent. Can students suggest what colour refers to each atom thinking about valency? It’s fun to challenge students to make C2H4 when teaching alkenes. Molymods do take up valuable teaching time so make sure there is sufficient bang (learning) for your buck (time). Handing out beakers, containing just a few sticks and balls relevant to the task, can help speed up activities.
Covalent and ionic bonding: what’s the difference?
GCSE activity and worksheet that uses models to compare ionic and covalent bonding. Students are presented with two images. The first shows two parrots eating a flower. The second shows a little boy stealing money from a handbag. Students describe how the two pictures represent ionic and covalent bonding. They can then create their own models. This resource was contributed by Shilpi Begum. (PDF)
Melting simple and giant covalent structures
GCSE activity and worksheet to help students observe what happens when we heat giant and simple covalent substances. Students use the interactive white board to pull apart models of simple and giant compounds. This is a powerful technique to show the difference between breaking bonds and pulling apart intermolecular forces. By asking students to do this on the board you can quickly see what they are thinking and provide feedback.
Graphite as a conductor
This is a fabulous demonstration showing how graphite, a carbon allotrope, can conduct electricity.
My little lecture demo, got me a round of applause ! (Suspect their standards for applause are quite low!) pic.twitter.com/A6mhATpoW4
— Dr Kristy Turner (@doc_kristy) November 13, 2018
- Bonding and physical properties
- Covalent bonding
- Intermolecular forces
- Ionic bonding
- Metallic bonding




